
【Detail】
Chemical Description
 ◆ Product name: Povidone K
◆ Product name: Povidone K
◆ Brand name: BOVIDONE® K
◆ CAS NO.:9003-39-8
◆ INCI Name: Polyvinylpyrrolidone (abbr. PVP)
◆ CEP Reg. No: 2014-372
DMF Reg. No: 29049
◆ Chinese Excipient Registration No. of BOVIDONE® K30: F20170000142
Physical and Chemical properties
-- Freely soluble in water and a variety of organic solvents;
-- Free-flowing, Hygroscopic powder, solubilization, physiological compatibility, high adhesive ability;
Typical Properties
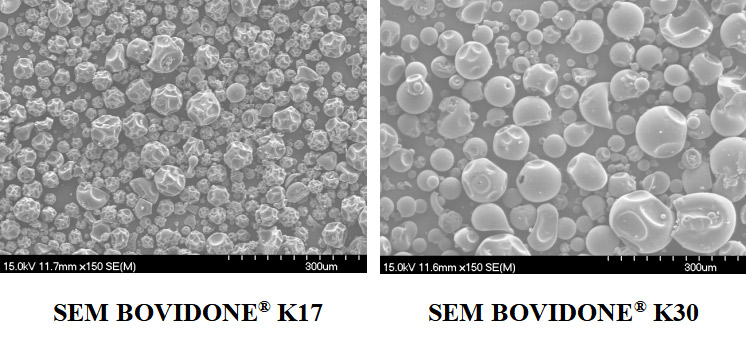
Specification
|
Product |
K-Value |
Mv. |
Impurity A (ppm) |
Peroxides (ppm) |
2-P(%) |
Element Impurity |
|
BOVIDONE® K15 |
12.8-17.3 |
4500-9700 |
≤10.0 |
≤400 |
≤3.0 |
Fulfil ICH Q3D |
|
BOVIDONE® K17 |
15.3-18.4 |
7100-11000 |
≤10.0 |
≤400 |
≤3.0 |
Fulfil ICH Q3D |
|
BOVIDONE® K25 |
22.5-27.0 |
19300 -31100 |
≤5.0 |
≤200 |
≤3.0 |
Fulfil ICH Q3D |
|
BOVIDONE® K30 |
27.0-32.4 |
31700-51400 |
≤5.0 |
≤150 |
≤2.0 |
Fulfil ICH Q3D |
|
BOVIDONE® K90 |
81.0-97.2 |
790000-1350000 |
≤10.0 |
≤400 |
≤3.0 |
Fulfil ICH Q3D |
Applications
◆ The optimum binder for solid preparations, suitable for wet granulation, dry granulation, direct pressing and fluidized bed granulation, especially for quick-release and anhydrous preparations, as well as for drugs with poor water solubity, water sensitivity and poor compressibility. It can effectively improve the compressibility of particles, improve the adhesion and hardness of tablets, and promote the release of drugs.
◆ Used as coating agent of active pharmaceutical ingredients enhance drug stability;
◆ Used as suspension stabilizer, increase the suspension stability of oral liquid preparation.
◆ Used as solubilizer to increase the solubility and bioavailability of insoluble drug preparations.
Packing and Storage
◆ Packed in 25kg or 50kg fiber or plastic drum, inner lined with double medicinal polyethylene film bag.
◆ Stored in dry place at room temperature.
.jpg)

